How respiratory signals are calibrated with DECRO solution?
By Leandro Fontana-Pires, PhD candidate
About Respiratory Inductive Plethysmography (RIP) and its principle
Because, respiratory function is a major subject of interest in a wide set of research fields (Pharmacology, Pathology, Infectiology, etc.), several technics have been developed for its monitoring. They rely on very different physical principles (pressure variations, optical movement, perimeter, etc.) all associated with drawbacks and advantages.
The DECRO jacket records respiratory volumes variations using electromagnetic sensors directly integrated in the jacket in a way to encompass the thorax and the abdomen of the animal (Figure 1-a). This technique, called Respiratory Inductive Plethysmography (RIP), is widely used to monitor respiratory function in humans and large animals. It has the advantage to be totally non-invasive and not requiring additional equipment that could influence the breathing pattern, such as mouth pieces, nose clips or face masks (Poole et al., 2000; Leutheuser et al., 2014).
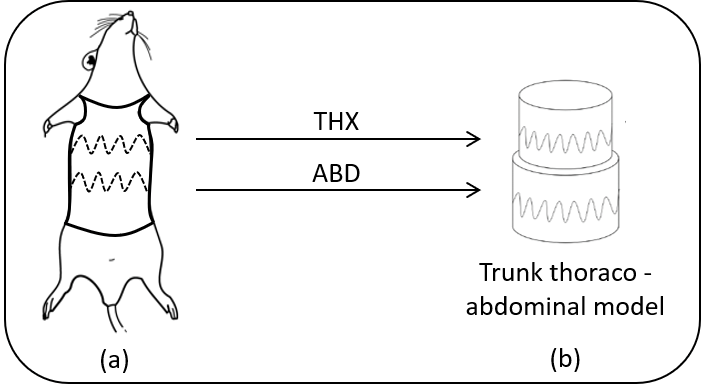
Figure 1 : (a) anatomical position of the coins for the implementation of a thoracic PCRI in rats and (b) the thoraco-abdominal model of the trunk (THX: Thorax, ABD: Abdomen).
RIP technique is based on an electromagnetic measurement of body surface displacement using inductive coils (Gerald Williams et al., 1994) with a very simple principle: as the subject breathes, the volume of his trunk changes, and with it a correspond change in its cross-section, which is detected by proportional variations in the inductance of the coils (Martinot-Lagarde et al., 1988).
In RIP, the respiratory volume or airflow variation is reconstructed by combining the signals from the two sensors together as demonstrated by (Konno and Mead, 1967) with the assumption that these two compartments (thorax and abdomen) move independently:
![]()
where THX and ABD are thorax and abdominal cross-sectional variations (in cm²) and ![]() and
and ![]() are individual calibration coefficients of each sensor, respectively.
are individual calibration coefficients of each sensor, respectively.
In other words, the trunk is modelled with two adjacent cylinders (Figure 1-b) and the section of each cylinder is measured by the sensors.
Why calibrate respiratory signals?
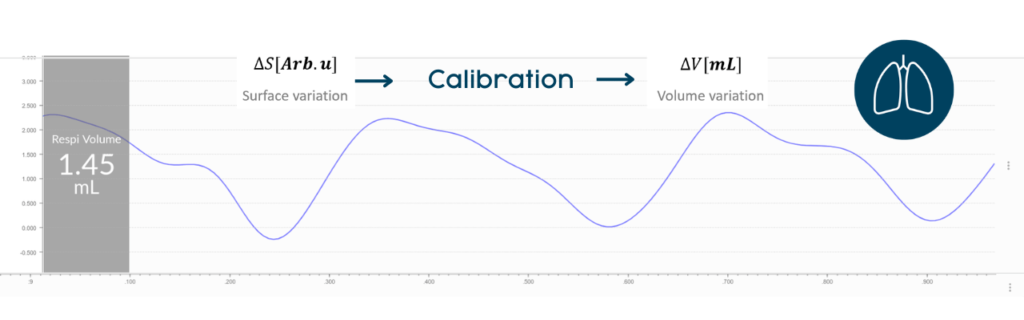
The signal provided by DECRO RIP is proportional to the variations of the trunk volume from cross-sectional variations of thorax and abdomen (Konno and Mead, 1967). This signal is expressed in cm² or arbitrary units (Arb.u) and suitable for relative variations evaluations (Martinot-Lagarde et al., 1988). However, using relative variations (or arbitrary units) may not be sufficient for certain situations. Thus, a method to transform (calibrate) the variations of thoracic and abdominal sections (expressed in Arb.u) into the variation of respiratory volume (mL) is required. This article will allow you to better understand how it is done with DECRO.

How to calibrate RIP in rodents?
Usually RIP sensors in humans and animals are individually calibrated before being combined together (Konno and Mead, 1967).
This calibration consists in determining and applying a calibration factor (gain) to each section variation signal (THX / ABD) in order to obtain a volume signal in mL (cm3). From dimensional point of view, it is interesting to note that this gain is equivalent to the height of each cylinder compartment (Figure 1-b) in cm. However, the determination of individual gains generally requires a direct and synchronized measurement of airflow with another method (Loveridge et al., 1983) (pneumotachograph, whole body plethysmograph, etc.) which is more difficult to implement in small animals such as rodents (Mortola and Frappell, 2013).
Alternatively, a global calibration of all the sensors can performed by equalizing the average tidal volume (Tv) or minute volume (Mv) measured over a chosen period with the expected Tv/Mv values for that same period. The expected Tv/Mv values can be obtained by different sources including a calibrated monitoring provided by another method (without the need for exact time synchronization) or prediction based on historical data on the model of the subject. This second approach is much easier to perform and this is the one proposed by DECRO.
Calibration methods proposed by DECRO
Based on the global calibration approach there are two different methods available in the software: automatic and reference measurement calibration.
Automatic calibration
Principle of automatic calibration: DECRO offers an automatic calibration method based on an estimation model determined by the Association of Inhalation Toxicologists (AIT) and validated by a reference organization – OECD (Alexander et al., 2008). They describe a new formula to calculate Mv, an important variable for the calculation of the delivered dose in animal aerosol inhalation toxicology studies with pharmaceuticals, based only on the animal’s body weight. For this, they used a total of 18 datasets of 10 laboratories from four species. Using this formula, it is possible to calculate Tv (Mv = Respiratory Rate * Tv).
The automatic calibration method propose by DECRO only requires two pieces of information:
- The subject’s body weight (used to estimate the Tv);
- A region in the signal where there is a mix in the state of the animal between rest and activity (used to measure the Tv).
The ratio between the estimated and measured Tv is calculated by the software and gives a gain capable of transforming into milliliters (mL).
To perform an automatic calibration in the software only a few steps are required. The first one is determining a region of the signal mixing activity and rest of the animal which is representative of its basal state. The duration can be between 30 min and 1 hour, prior the start of the experiment.
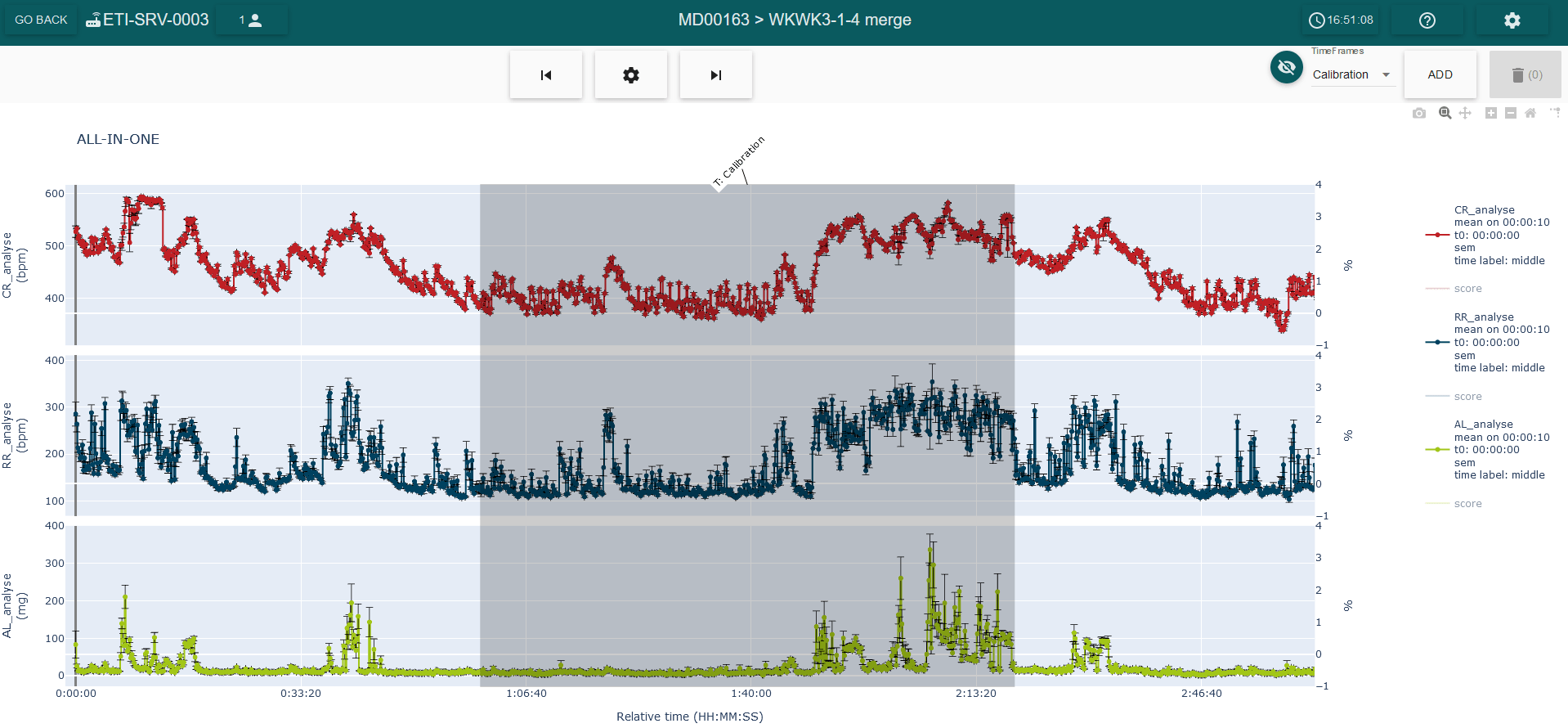
Figure 2: screenshot of the LASA software from the ALL-IN-ONE view, where a calibration timeframe mixing activity and rest of the animal is defined. It’s possible to observe that the timeframe chosen starts 1h after the animal has been equipped.
The second step is to enter the animal’s body weight (if it has not been loaded automatically) and perform the automatic calibration. The purpose of this mode is to calibrate the RIP signals quickly and easily.
As a result, two variables are created in the software corresponding to calibrated Tv and Mv, expressed in mL (Figure 3). The calibration timeframe used for this calibration can also be displayed (shaded area).
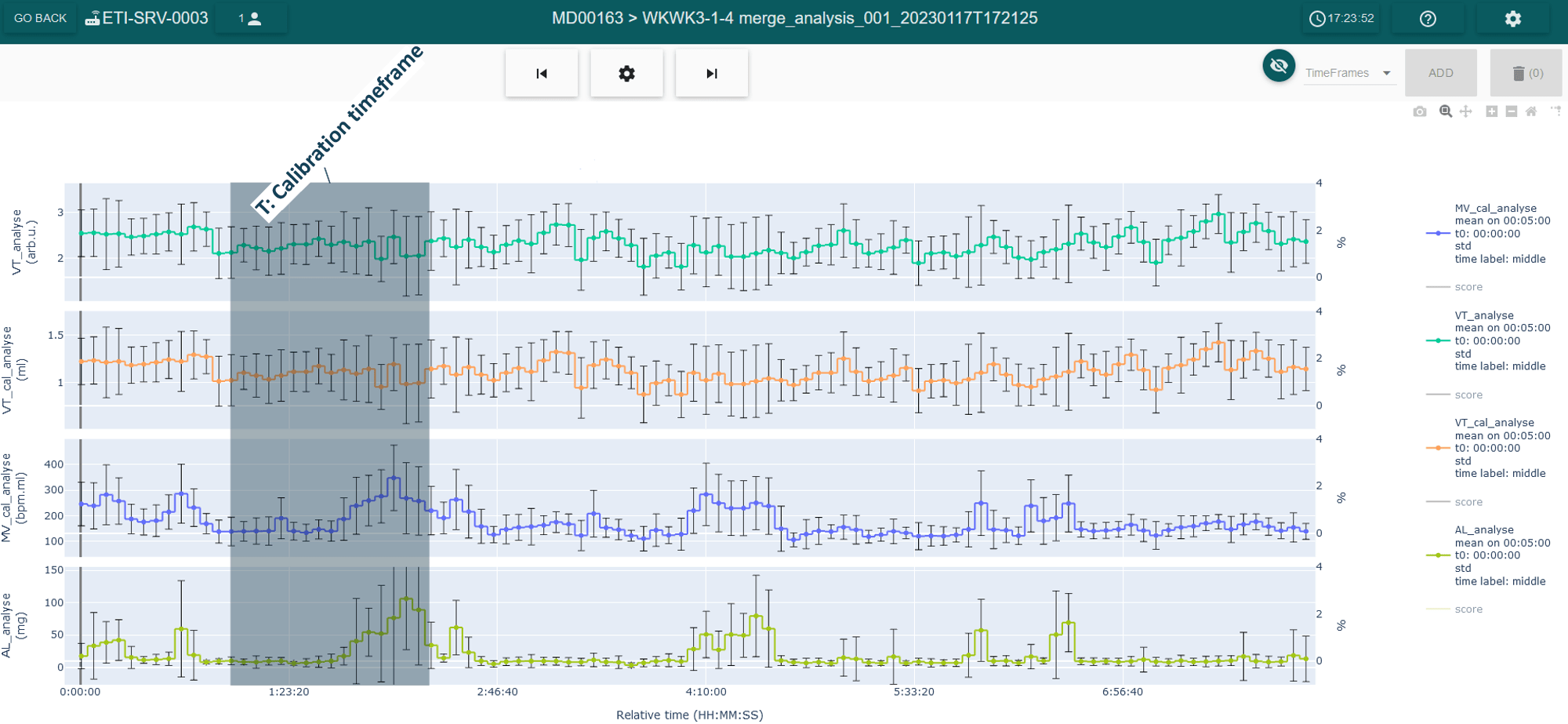
Figure 3: screenshot of the LASA software from the analysis view, where the Tv (Arb.u), Tv (mL), Mv (mL) and AL (mg) are displayed from top to bottom. The calibration timeframe is also displayed as the shaded area in the figure.
Validation of automatic calibration: Tv measured with DECRO and calibrated using this method have been favorably compared to a simultaneous reference measurement using an unrestrained whole-body plethysmography chamber (UWBP) in 7 rats (male Han Wistar rats from 296-314g). Animal were recorded during 90 min with the two systems and data were compared using the Bland-Altman (B&A) diagram (which is very commonly used to assess the agreement between two systems (Martin Bland and Altman, 1986)). According to the Bland-Altman (B&A) diagram depicted in the Figure 4, the two methods have an agreement of +/- 68% and no constant bias is found between the Tidal volume measurement, which means that the method used for calibration (DECRO automatic calibration) results in values close to those of the reference method (UWBP).
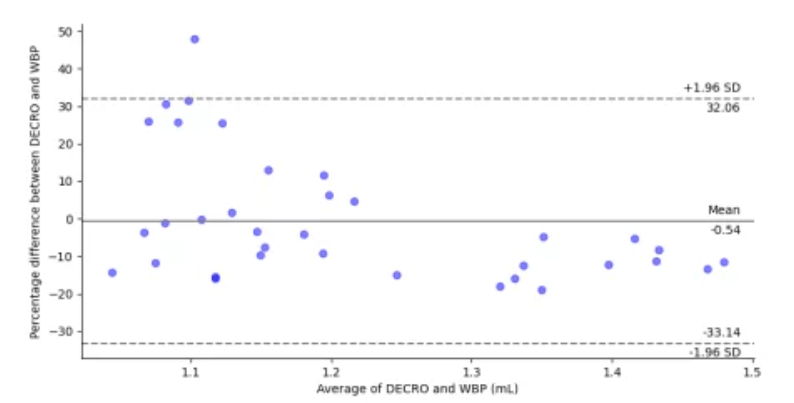
Figure 4: Bland-Altman diagram of tidal volume (Tv) variation measured with DECRO and calibrated with the automatic method calibration and the reference measure of Tv acquired with a whole-body plethysmography (WBP). The difference is expressed in percentage and the average in milliliters (mL). The dashed lines represent the concordance limits (1.96![]() -95%) and the solid line represents the mean difference between methods.
-95%) and the solid line represents the mean difference between methods.
Calibration with a reference measurement
Principle of calibration with a reference measurement: for those who don’t want to use the model to estimate the Tv, can use another measurement device as reference to the expected Tv, for example an UWBP. In this case, the animal should be dressed with the DECRO jacket and then placed into the UWBP. Then, Tv is measured in the region where the animal was simultaneously recorded on both devices or alternatively where the animal is considered in similar condition.
The calibration process is similar to the first method, with the difference that the expected Tv is known. Thus, two things are needed to use this method:
- A reference Tv measured from another monitoring device;
- A region in the signal where the subject has been simultaneously recorded with the two devices or alternatively where the animal is considered in similar condition (used to measure the Tv).
The calibration factor (gain) is then calculated, being the ratio between the reference (UWBP) and measured (DECRO) Tv.
Validation of calibration with a reference measurement: this method of calibration has been validated by comparison with the WBP (Flenet et al., 2020) and comparable to previous validation conducted in anesthetized rats to validate the respiratory monitoring against invasive pneumotachograph (PNT) recording (Flénet et al., 2017).
In summary
As described in this article, RIP is a well-known technique for functional respiratory monitoring that requires calibration to express respiratory variables, such as Tv and Mv, in terms of unit volume (typically mL). However, most existing calibration methods make use of a reference system and, considering the challenge of achieving time synchronization between systems in everyday laboratory procedures or considering the disadvantage of needing to have a reference system for calibration, we have developed alternative calibration methods.
Both developed methods are easy and quick to use with DECRO system, and have their advantages. The automatic calibration method is a simple calibration process that does not require the use of any external equipment, such as face masks, or any kind of restraint on the animal, requiring minimal information. The reference measurement calibration method, on the other hand, allows to use another system to calibrate the DECRO jacket without the need for real-time synchronization.
Each method has been validated and provides reliable results as shown by the comparative studies presented in this article.
References
Alexander, D.J. et al. (2008) ‘Association of Inhalation Toxicologists (AIT) Working Party Recommendation for Standard Delivered Dose Calculation and Expression in Non-Clinical Aerosol Inhalation Toxicology Studies with Pharmaceuticals’, Inhalation Toxicology, 20(13), pp. 1179–1189. Available at: https://doi.org/10.1080/08958370802207318.
Flénet, T. et al. (2017) ‘High-resolution respiratory inductive plethysmography in rats: validation in anesthetized conditions’, Physiological Measurement, 38(7), pp. 1362–1372. Available at: https://doi.org/10.1088/1361-6579/aa6737.
Flenet, T. et al. (2020) ‘Assessment of cardiorespiratory function using telemetric jacket in rodents’, Journal of Pharmacological and Toxicological Methods, 105, p. 106824. Available at: https://doi.org/10.1016/j.vascn.2020.106824.
Konno, K. and Mead, J. (1967) ‘Measurement of the separate volume changes of rib cage and abdomen during breathing’, Journal of Applied Physiology, 22(3), pp. 407–422. Available at: https://doi.org/10.1152/jappl.1967.22.3.407.
Leutheuser, H. et al. (2014) ‘Comparison of a priori calibration models for respiratory inductance plethysmography during running’, in 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, pp. 6393–6396. Available at: https://doi.org/10.1109/EMBC.2014.6945091.
Loveridge, B. et al. (1983) ‘Single-position calibration of the respiratory inductance plethysmograph’, Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology, 55(3), pp. 1031–1034. Available at: https://doi.org/10.1152/jappl.1983.55.3.1031.
Martin Bland, J. and Altman, DouglasG. (1986) ‘STATISTICAL METHODS FOR ASSESSING AGREEMENT BETWEEN TWO METHODS OF CLINICAL MEASUREMENT’, The Lancet, 327(8476), pp. 307–310. Available at: https://doi.org/10.1016/S0140-6736(86)90837-8.
Martinot-Lagarde, P. et al. (1988) ‘What does inductance plethysmography really measure?’, Journal of Applied Physiology, 64(4), pp. 1749–1756. Available at: https://doi.org/10.1152/jappl.1988.64.4.1749.
Mortola, J.P. and Frappell, P.B. (2013) ‘Measurements of air ventilation in small vertebrates’, Respiratory Physiology & Neurobiology, 186(2), pp. 197–205. Available at: https://doi.org/10.1016/j.resp.2013.02.001.
Poole, K.A. et al. (2000) ‘Respiratory inductance plethysmography in healthy infants: a comparison of three calibration methods’, European Respiratory Journal, 16(6), pp. 1084–1090.
